Medicinal chemistry
Almost all projects in the lab are based on the synthesis of organic molecules with new architecture and/or new biological function.

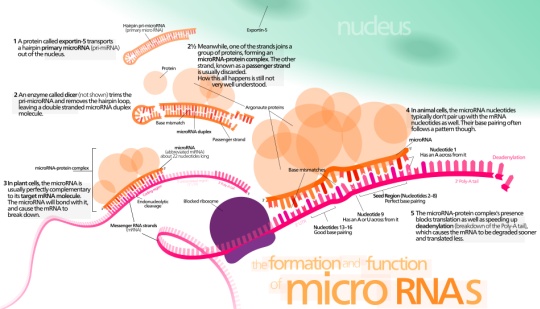
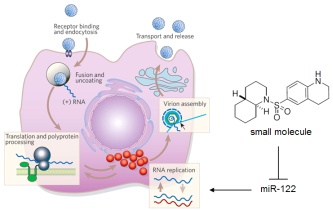
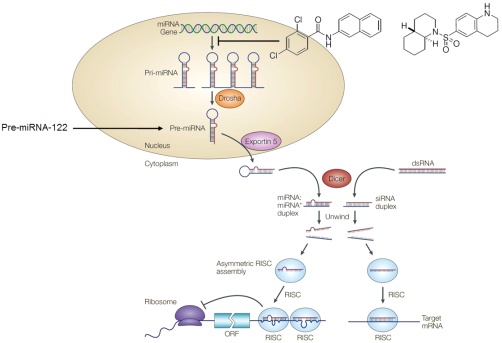
We are developing small molecules that target the microRNA pathway. MicroRNAs (miRNAs) are single-stranded noncoding RNAs of approximately 22 nucleotides that represent a new class of gene regulators. It is estimated that >30% of all genes and almost all genetic networks are, in part, controlled by miRNAs. Cancer, cardiovascular disease, and several viral infections have all been linked to the presence of specific miRNAs. Thus, developing small molecule modifiers for the miRNA pathway will have significant therapeutic implications. Click on the image on the right to learn more about the miRNA pathway.

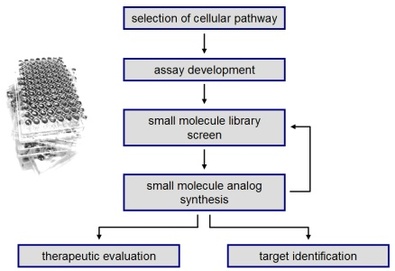
We are following a drug discovery approach that starts with the selection of a cellular pathway that is involved in human disease, followed by the development and validation of a reporter assay for small molecule screening. Libraries of compounds are assayed and analogs of hit compounds are synthesized in order to improve their activity and to learn about their structure-activity relationship. The obtained small molecule probes are then used to validate the initial pathway as a therapeutic target and to further study its biological and physiological implications.

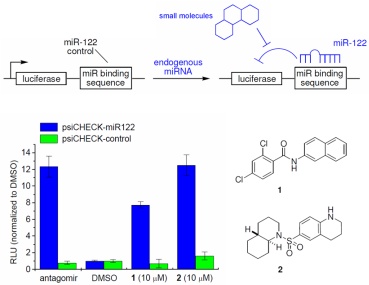
We have successfully applied this approach to the discovery of the first small molecule inhibitors of the miRNA pathway. For example, we developed a cellular assay for miR-122 function, conducted a pilot screen followed by a high-throughput screen, and have identified two inhibitors, the amide 1 and the sulfonamide 2, who efficiently silence miR-122 function. We selected miR-122 as a target, since it is the most abundant miRNA in the liver and is involved in hepatitis C virus infection and other liver diseases.

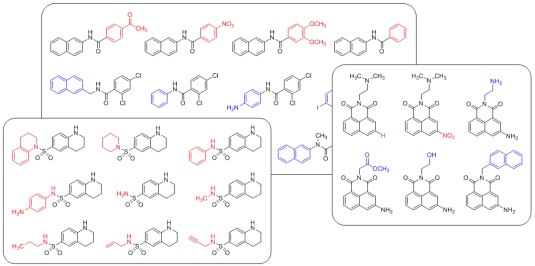
All discovered hit compounds are rigorously tested in several secondary assays in order to confirm their activity. In addition, we apply our synthetic chemistry expertise in extensive medicinal chemistry efforts to systematically modify the identified structures, often requiring the development of new synthetic approaches in order to facilitate the construction of analog collections. The goal is to obtain a detailed picture of the molecular requirements of each inhibitor for optimal function. In addition, we are converting those inhibitors into molecular probes by introducing functional handles for target identification.

The best compounds are evaluated for their potential therapeutic activity. In case of our miR-122 inhibitors, we are conducting hepatitis C virus replication studies, since miR-122 was shown to play a very important role in the life cycle of the virus. Importantly, our sulfonamide 1 was able to reduce viral RNA loads in human liver cells by 90% and thus represents a fundamentally new approach to the treatment of HCV infection.

We are also applying our discovered inhibitors as molecular probes to investigate the regulation and biogenesis of miRNAs. We have already identified that our miRNA inhibitors act on the transcriptional level by either directly inhibiting miRNA expression or by modulating the regulation of their transcription. Supplying a downstream component of the pathway, pre-miRNA-122, eliminates the inhibitory activity of our compounds, further validating that they act on early steps of the pathway. We are currently using our structure-activity knowledge of these molecules in order to generate probes that can be used for the identification of the actual protein targets of these miRNA inhibitors.