Optochemical biology

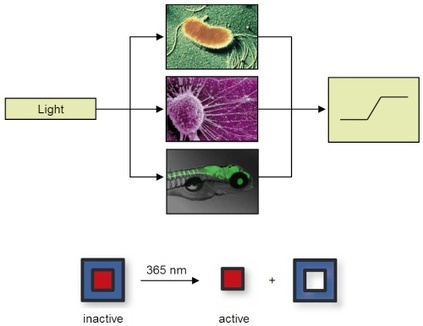
Nature controls biological processes, for example, signal transduction, protein function and gene expression, with exquisite spatial and temporal precision. This is particularly visible during embryo development (see the first 48 hours of zebrafish development in the movie on the right). In order to study and understand these processes, an equally precise control element is required. Light is an excellent tool for this purpose, as it can be precisely regulated in timing, location, wavelength, and amplitude, thereby enabling high-resolution optical control of biological processes. The developments of optogenetic tool sets are revolutionizing many aspects of science, most importantly neurobiology. We are developing related optochemical tools that are based on our ability to combine synthetic chemistry with molecular, cell, and animal biology.

We are developing tools that enable optochemical control of protein, RNA, and DNA function in bacterial and mammalian cells, as well as multicellular model organisms, thus eliciting a defined output signal from the biological system under study by using light as an input signal. All tools are based on a fundamental concept: the blocking of essential functional groups on biologically active molecules with light-removable protecting groups, so called "caging groups". These caging groups are site-specifically installed, rendering the biological target completely inactive, until they are removed through illumination with UV, visible, or IR light. Since light can be controlled spatially and temporally, the activity of the caged molecule can be precisely controlled in location and timing.

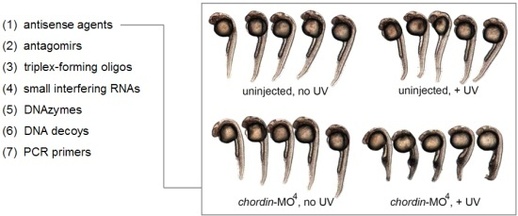
In order to achieve light-control of oligonucleotide function, we are developing synthetic approaches to monomeric building blocks for oligonucleotides that contain caged nucleobases. The caging groups block Watson-Crick hydrogen-bonding and completely prevent oligonucleotide duplex formation, until removed through a brief exposure to UV light. When the caging groups are installed on antisense oligonucleotides, they enable optochemical control of ON to OFF and OFF to ON switching of gene expression with unprecedented spatial and temporal resolution in human cells, tissues, and multicellular organisms. When bioconjugated to delivery agents, the caging groups enable targeted delivery of oligonucleotides in addition to spatio-temporal control.

The optochemical control of oligonucleotide function has been extended beyond standard RNA and DNA molecules to phosphorothioates, 2' OMe oligonucleotides, and morpholino oligomers. The latter haven been successfully used to light-control gene function in zebrafish and Xenopus embryos. In addition to antisense agents, our nucleobase-caging approach has been applied to the control of microRNA function with antagomirs and gene function with triplex-forming oligonucleotides, DNA decoys, and siRNAs. Caged DNAzymes have enabled the control of biochemical reactions, DNA computation has been optochemical triggered with caged input strands, and the polymerase chain reaction has been regulated with caged primers.

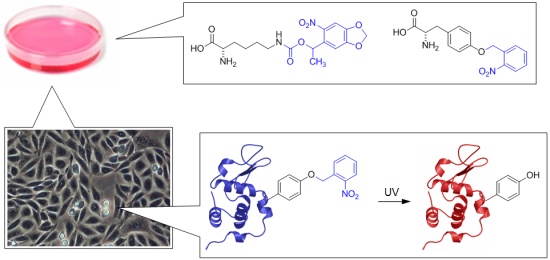
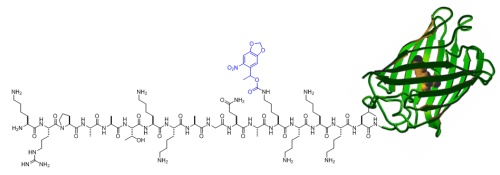
In order to site-specifically introduce caging groups onto amino acid residues in proteins, we appliy a synthetic biology approach using cells engineered with an expanded genetic code. The cells are grown in the presence of synthesized caged amino acids, e.g., caged lysine or caged tyrosine, and introduce those amino acids exclusively at amber stop codon mutations in the protein of interest. The protein containing the caged amino acid, typically in an active site location, is expressed inside the cells and is inactive until brief exposure to noninvasive UV light removes the caging group and activates protein function. The cellular process placed under optochemical control can then be studied with high spatial and temporal resolution.

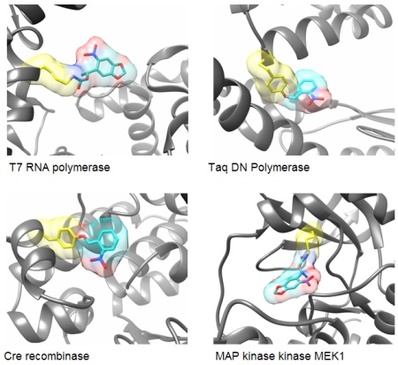
In order to render proteins responsive to light, we are incorporating a single photocaging group at a defined amino acid residue that is crucial for protein activity. This residue is identified based on mechanistic data that has been reported in the literature or generated by us. Alternatively, sites are selected based on structural information that allows us to generate hypotheses regarding the impact that a caging group would have on protein activity. For example, we have optochemically controlled the function of T7 RNA polymerase, Taq DNA polymerase, Cre recombinase, and the kinase MEK1, by blocking an essential active site tyrosine or lysine residue with a photocaging group. Importantly, the enzymes were completely inactive until illumination with UV light removed the caging groups.

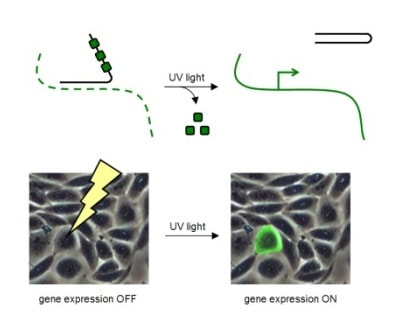
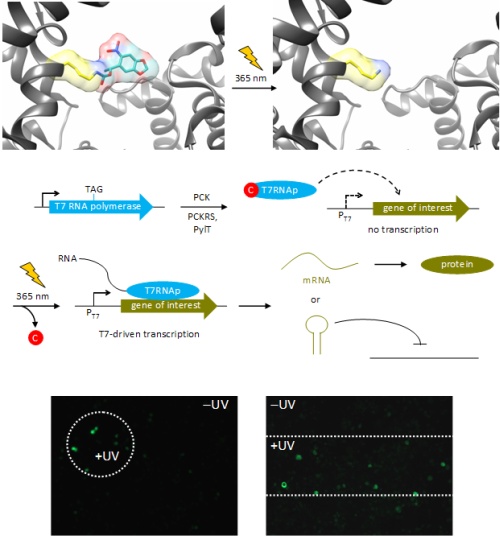
For example, an optochemically controlled T7 RNA polymerase was constructed by blocking access to the active site for incoming nucleotide triphosphates through installation of a caging group on a crucial lysine residue. The genetically encoded light-controlled enzyme was completely inactive when expressed in mammalian cells, until a brief UV illumination removed the caging group, thereby allowing access to the active site and activating RNA polymerization. By engineering cells that express the caged polymerase with genes or non-coding RNA sequences under control of a T7 promoter, we were able to achieve optochemical gene activation and light-triggered RNA interference. This enabled spatial control of gene function in mammalian cells, as shown for the localized activation of GFP in mammalian tissue culture. Importantly, only cells that were exposed to UV light display activation of protein expression.

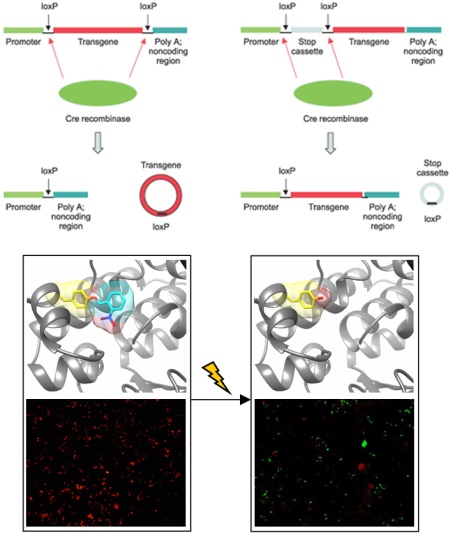
Cre recombinase is a topoisomerase isolated from bacteriophage P1. It has found widespread application in the genetic engineering of cells and multicellular model organisms, in particular mice. Cre recombinase recognizes so called loxP sites, partially palindromic sequences of 34 base pairs, in double-stranded DNA. Based on the directionality of the loxP sites, Cre excises or inverts the DNA between them. This Cre/lox system has been cleverly engineered to allow for activation and deactivation of gene expression in a wide range of biological systems. We developed optochemical control of Cre-mediated DNA recombination by placing a light-removable caging group onto an active site tyrosine residue. With the caging group in place, the tyrosine cannot undergo a nucleophilic attack onto the DNA phosphodiester backbone, thus rendering the enzyme completely inactive. Exposure to UV light removes the caging group and activates the Cre recombinase. This enabled us to optochemically control both gene activation and gene deactivation in mammalian cells.

Using genetic code expansion, we are able to achieve optochemically controlled protein translocation, for example, from the cytoplasm to the nucleus. A single lysine residue was replaced with a photocaged lysine in a nuclear localization sequence that was appended to green fluorescent protein. The presence of the caging group blocked activity of the nuclear localization sequence, leading to an even distribution of the protein in human cells engineered with an expanded genetic code. However, a brief illumination with light of 365 nm induced caging group removal and complete translocation of the protein into the nucleus within 60 seconds. Due to the defined starting point of this process, the kinetics of the active transport into the nucleus can be precisely determined.